Technologies
Accurate Pulse Oximetry for All Skin Tones
Masimo SET® Measures Accurately on Varying Skin Pigmentations
As a global leader in medical technologies, Masimo is committed to providing products that serve all people, regardless of age, gender, race, ethnicity, or skin tone.
Masimo SET® pulse oximetry has been shown to perform accurately on all skin pigmentations1-4 and Masimo continues to strive for equitable care for all by partnering with researchers, regulatory agencies, and humanitarian organizations.
Unbiased performance
between black and white skin tones with Masimo SET® pulse oximetry, as shown by peer-reviewed clinical research.1-4
200+ million
patients
are monitored by Masimo SET® pulse oximetry each year.5
All top 10
U.S. hospitals
use Masimo SET® as their primary pulse oximetry technology.6
Unrivaled
SpO2 accuracy
with RD SET® pulse oximetry sensors, achieved through continued innovation.7
Advancing Health Equity with Masimo SET® Pulse Oximetry
Masimo SET® pulse oximetry has been developed and tested using nearly equal numbers of individuals with dark and light skin (exceeding FDA requirements), with the goal of eliminating bias due to skin pigmentation.2
Learn more about Masimo SET® technologyContinued Innovation
Masimo has made strides in improving accuracy with the latest RD SET® pulse oximetry sensor line, which offers best-in-class SpO2 accuracy specifications for all patients regardless of skin tone.7
Discover the RD SET sensor portfolioProven Performance
Research has shown there is no clinically significant difference in SpO2 bias between dark and light skin with RD SET sensors, even in low perfusion.3,4
See the clinical evidenceThe Work Continues
Masimo recognizes there is more work to be done in achieving health equity, so we challenge all medical device manufacturers to raise the bar and welcome clinicians to partner with Masimo to advance research on this topic.
Help us move research forwardMasimo’s Role on the FDA Advisory Panel
Masimo applauds the Food and Drug Administration (FDA) for convening an anesthesia panel to discuss improvements to premarket requirements related to skin pigmentation and health ethnicity.8
As a leading pulse oximetry manufacturer, Masimo has advocated for and continues to advocate for the following requirements for 510(k) clearance of pulse oximeters:
- Increased ARMS* accuracy performance specifications.
- Greater diversity of skin tones and larger sample sizes for validation studies.
- Improved skin pigmentation classification methods.
More Stringent Accuracy Requirements
Masimo has been proactive in raising the standard for health equity in pulse oximetry ahead of any regulatory requirement updates.
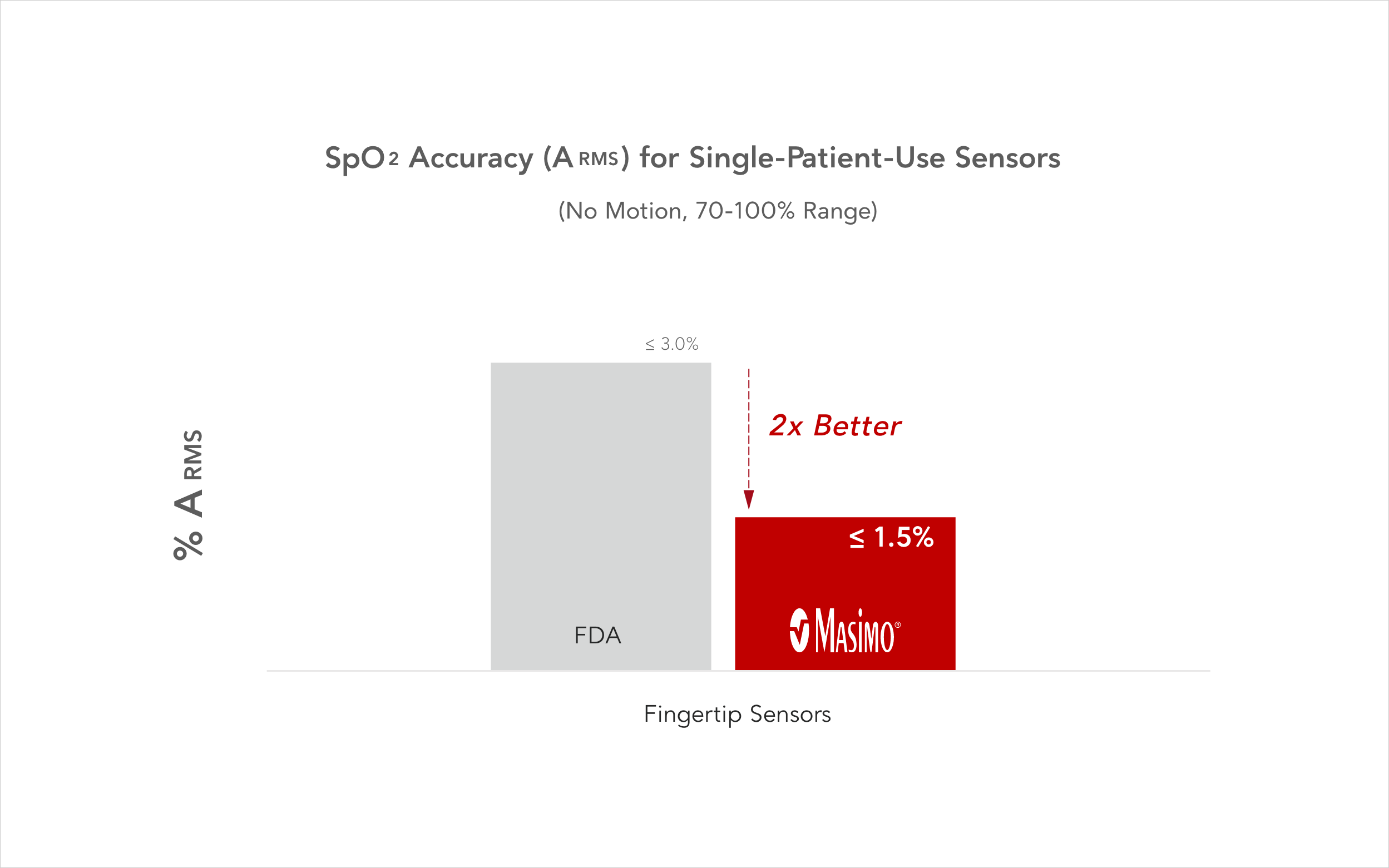
This chart depicts the FDA’s guidance on the typical SpO2 accuracy specification (ARMS) between measured (SpO2) and reference (SaO2) values under normal, non-motion conditions ranging from 70% to 100% SpO2.9
In comparison, Masimo RD SET single-patient-use pulse oximetry sensors exceed FDA guidance on accuracy. Masimo advocates for making the requirements more stringent to ensure better patient care for all.7,10-12
Greater Diversity of Skin Tones in Study Subjects
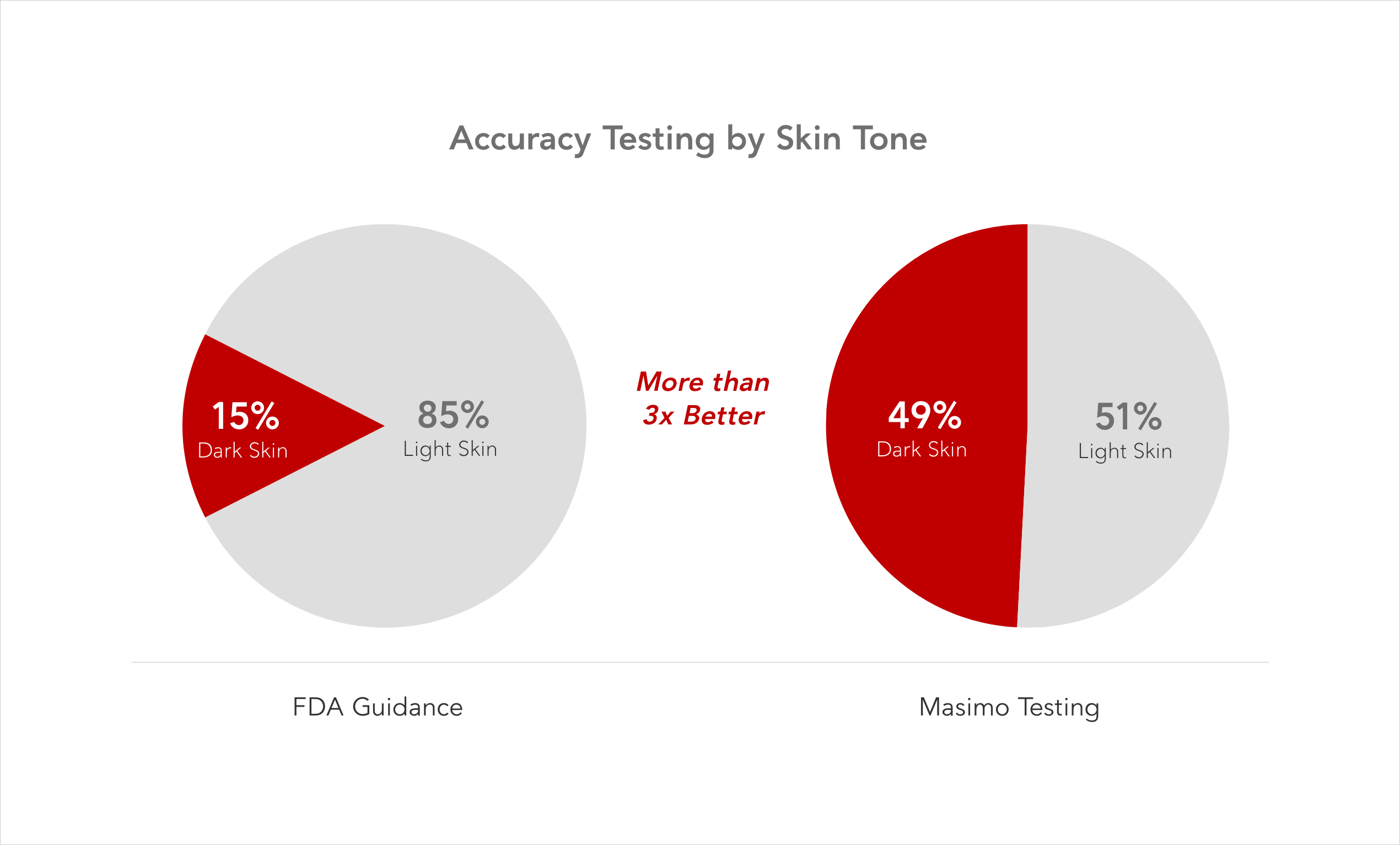
FDA guidance requires at least 2 darkly pigmented subjects or 15% of the subject pool, whichever is larger, for pulse oximetry testing.8
Masimo continues to aim for nearly equal numbers of subjects with dark and light skin in the development and testing of SET® pulse oximetry. Historically, Masimo testing has exceeded FDA requirements by more than 3 times, increased sample sizes, and aimed for a 50-50 split between subjects with dark and light skin. Masimo advocates for industry to join us in increasing the minimum number of dark skin pigment subject in their validation testing to ensure equitable performance across varying skin tones.3
More Inclusive Skin Pigmentation Measures
Masimo recommends using a standardized pigment categorization like the 10-point Massey-Martin Scale or Monk Scale, which are more objective methods for estimating skin pigmentation than the Fitzpatrick Skin Type Scale or racial/ethnic self-identification, when available.8
While these 10-point scales are still subjective, the FDA has reported that they have lower variance due to the use of standardized color categories.1,8
Read the FDA Executive Summary to learn the pros and cons of various assessment methods
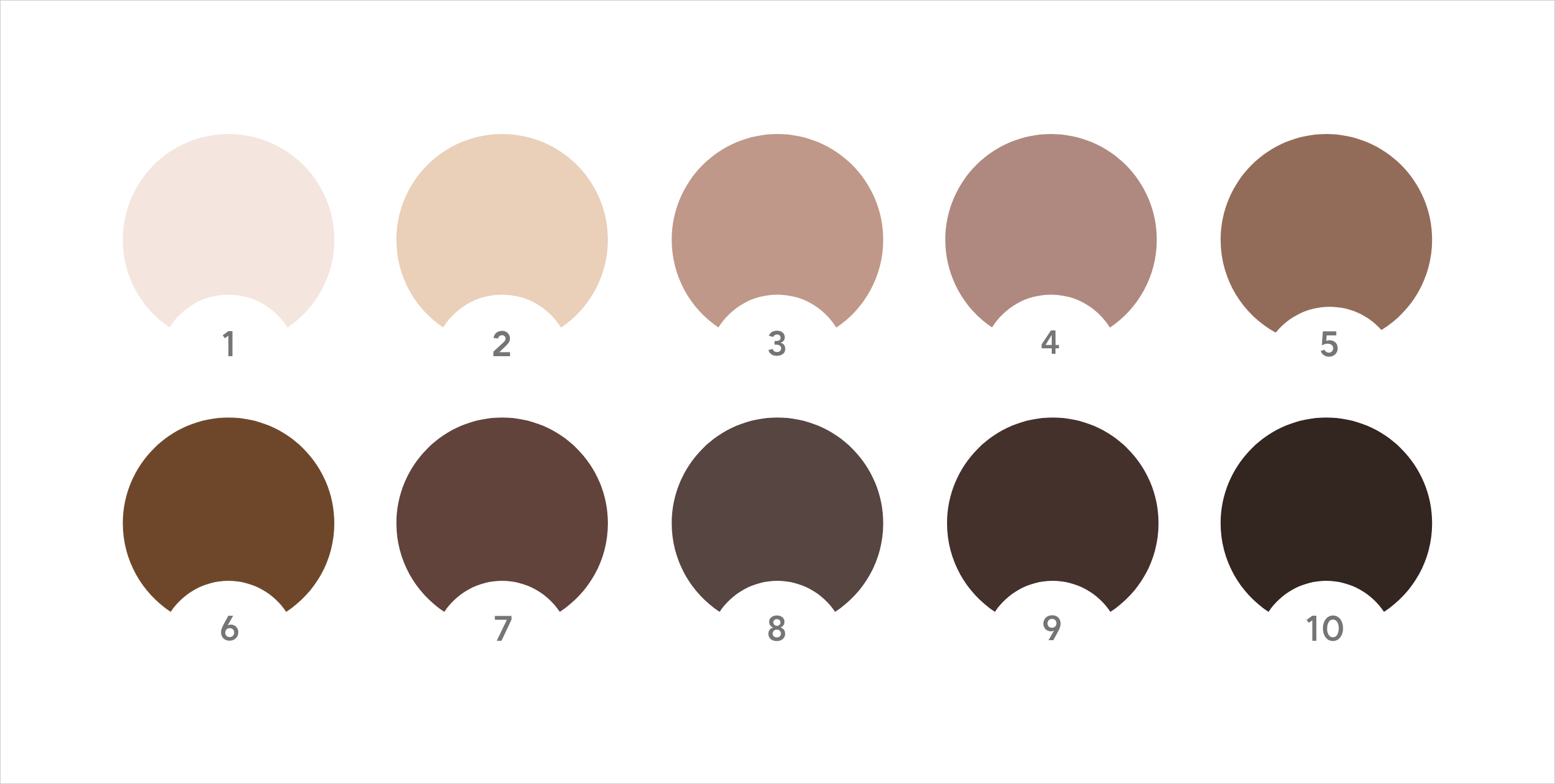
Massey-Martin Skin Color Scale
Stories From Around the World
Saving Lives
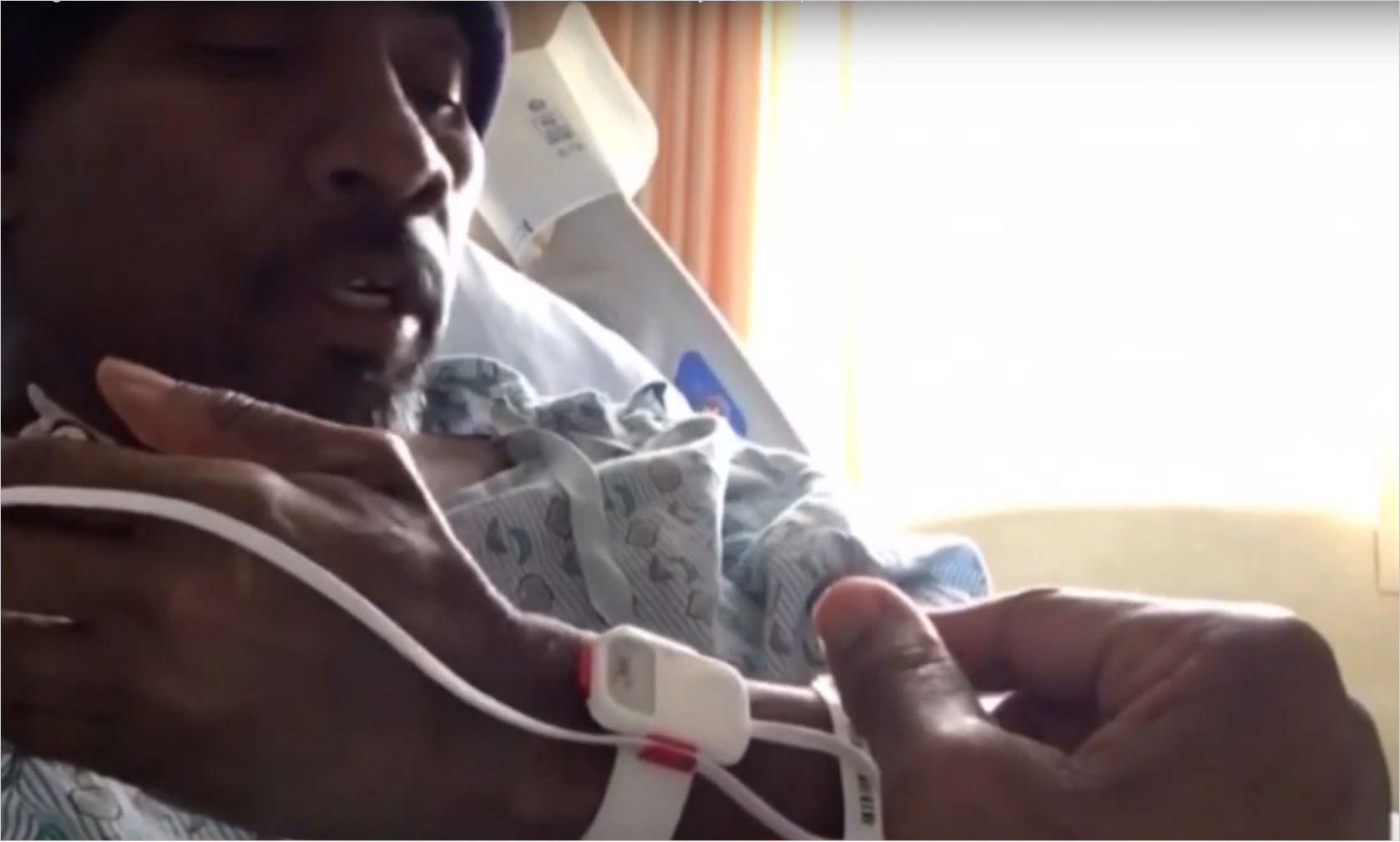
“This right here saved my life and that’s why I’m here today.”
- Leonardo Frazier | Patient at University Hospitals monitored by Masimo SET® technology
Serving a Global Community
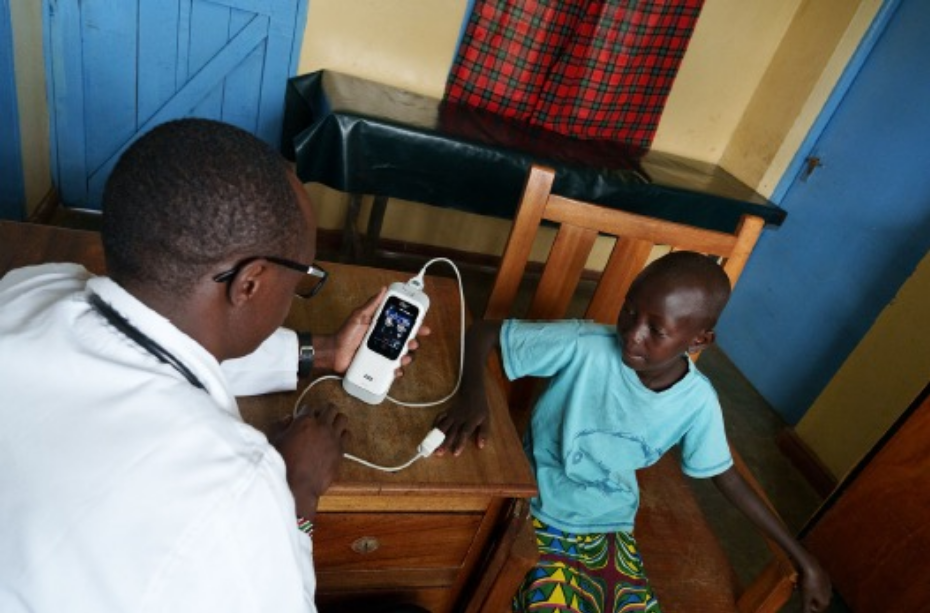
Clinician using Masimo SET® pulse oximetry to monitor a child in Kenya
More than five-billion people do not have access to safe and affordable surgical care when they need it the most.13
Even when care is available in underserved communities, healthcare providers and their patients often have limited access to accurate, reliable, and effective patient monitoring tools.
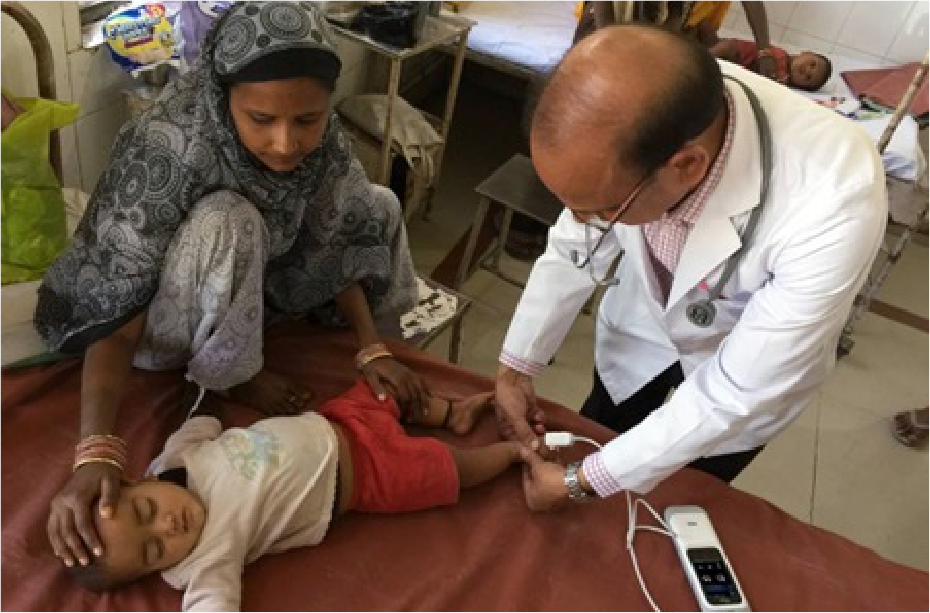
Clinician using Masimo SET® to monitor an infant in India
Masimo contributes to health equity with pulse oximetry technology that has been shown to measure accurately on all skin tones.1-3
Through its Global Health division, Masimo provides major humanitarian organizations, such as the World Health Organization (WHO), UNICEF, Pan American Health Organization (PAHO), World Bank, Global Fund, and a range of international non-governmental organizations (iNGOs), direct access to patient monitoring technologies.
Inspiring Confidence
Masimo’s efforts have been supported by independent surveys of users and administrators across health facilities in regions serving patients with a prevalence of dark skin, who report strong confidence in the SpO2 accuracy of Masimo SET® based on their experiences.14
How to Help Advance Research
Considerations for Studies Comparing Different Pulse Oximetry Manufacturers

- Use optical shielding with an opaque material, because multiple sensors in proximity can interfere with each other’s performance.
- Use sensors on the same application site, as the unique physiology of each location (e.g. finger vs. ear) can lead to variations in SpO2 accuracy.7,15,16
- Take note of the makes and models of pulse oximeters used and accuracy specifications for sensors used, as the performance of different pulse oximetry devices and sensors can vary.
- Ensure consistent application of warming or cooling procedures to the extremities for each pulse oximetry sensor, if applicable.
- Use comparable averaging time and sensitivity mode settings for the devices.
- Use a statistically valid method for handling outlier data and define similar cutoffs for each technology.
While these are all important considerations to factor into how the research is conducted, it is equally important to document these details in manuscripts and final publications to help others make informed decisions.
Masimo invites further discussion.
Peer-reviewed Clinical Evidence
Accuracy in Varying Skin Pigmentation
Clinicians and patients can be confident in the performance of Masimo SET® pulse oximetry technology, regardless of skin tone. The featured literature has been peer-reviewed and confirms the high-quality performance of Masimo SET® in varying skin pigmentation.
Clinical Evidence
“Performance of pulse oximeters as a function of race compared to skin pigmentation: a single center retrospective study”
Marlar AI, et al. J Clin Monit Comput. 2024 Aug 28.
- The authors reviewed Masimo SET® SpO2 data and reported that “this retrospective, single institution review did not find a clinically significant difference in the accuracy of pulse oximeters with respect to skin pigmentation.”1
“Racial Effects on Masimo Pulse Oximetry: Impact of Low Perfusion Index”
Sharma et al. J Clin Monit and Comput. 2024;38(2):347-354.
- The authors concluded that “Masimo SET® pulse oximeters with RD SET® sensors are accurate for individuals of both Black and White races when Pi [perfusion index] is normal, as well as during conditions when Pi is low.”3
“Racial Effects on Masimo Pulse Oximetry: A Laboratory Study”
Barker SJ, Wilson WC. J of Clin Monit and Comput. 2022;37,567-74.
- The authors concluded that “Masimo RD SET® pulse oximeter sensors showed an absence of clinically significant differences in accuracy between Black and White subjects.”2
“The Effect of Skin Pigmentation on the Accuracy of Pulse Oximetry in Infants with Hypoxemia”
Foglia EE, et al. J Pediatr. 2017 Mar;182:375-377.e2.
- The authors concluded that “There was no significant difference in systematic bias based on skin pigment for either oximeter,” and reported that “Mean Nellcor bias was significantly greater than Masimo bias, P = .0005.”4

“Masimo believes every individual is entitled to equitable patient care. As a leader in pulse oximetry, we advocate for raising the industry standard for all manufacturers. We recognize there is more work to be done, and we look forward to collaborating with researchers on real-world studies to deepen our understanding and continue enhancing our technology for a diverse global patient population.”
- Daniel Cantillon, MD | Chief Medical Officer, Masimo
Raising the Standard with Masimo SET®



Resources
Need more information? Below are key resources about Masimo SET®.
Web Pages
Videos
Set Up Your Risk-Free Trial
Contact us today for more information on how Masimo SET® can make a difference for your healthcare organization.
Marlar AI, et al. J Clin Monit Comput. 2024 Aug 28.
Barker SJ, Wilson WC. J Clin Monit Comput. 2023;37:567-574.
Sharma V, et al. J Clin Monit and Comput. 2024 Jan;38:347-354.
Foglia EE, et al. J Pediatr. 2017 Mar;182:375-377.e2.
Estimate: Masimo data on file.
As ranked in the 2024 Newsweek World’s Best Hospitals listing:
https://www.newsweek.com/rankings/worlds-best-hospitals-2024/united-states.Masimo RD SET Sensor Instructions for Use: LAB-10497A posted on https://techdocs.masimo.com/.
FDA. Executive Summary: Performance Evaluation of Pulse Oximeters Taking into Consideration Skin Pigmentation, Race, and Ethnicity. February 2, 2024.
FDA. Pulse Oximeters. – Premarket Notification Submissions [510(k)s]. Guidance for Industry and Food and Drug Administration Staff. March 4, 2013.
Masimo RD SET DCI Sensor Instructions for Use: LAB-8994B posted on https://techdocs.masimo.com/.
Masimo RD SET E1 Sensor Instructions for Use: LAB-9025B posted on https://techdocs.masimo.com/.
Masimo RD SET TFA-1 Sensor Instructions for Use: LAB-9508B posted on https://techdocs.masimo.com/.
Ng-Kamstra JS et al. BMJ Global Health. 2016;1:e000011.11
Masimo Data on File: Tools for Integrated Management of Childhood Illness (TIMCI) Post-market Landscape on Masimo Rad-G Pulse Oximeter Device. May 2023.
Nellcor OxiMax Sensor Instructions for Use: PT00053377B, PT00053381B, and PT00053384B posted on
www.medtronic.com/covidien/en-us/support/productmanuals.htmlMasimo LNCS DCI and TC-I Sensor Instructions for Use: LAB-4888G and LAB-5357G posted on https://techdocs.masimo.com/
* ARMS accuracy is a statistical calculation of the difference between device measurements and reference measurements. Approximately two-thirds of the device measurements fell within ± ARMS of the reference measurements in a controlled study.
For professional use. See instructions for use for full prescribing information including indications, contraindications, warnings and precautions. Caution: Federal (USA) law restricts this device to sale by or on the order of a physician.
PLCO-007463/PLM-14007B-1024