About
The Masimo Story
Masimo (NASDAQ: MASI) is a global medical technology company that develops and produces a wide array of industry-leading monitoring technologies, including innovative measurements, sensors, and patient monitors. Powered by the Masimo Hospital Automation™ platform, Masimo connectivity, automation, and telehealth and telemonitoring solutions are improving and automating care delivery both in the hospital and beyond. In addition, Masimo is home to an expanding array of consumer health and wellness solutions like the Masimo W1® health tracking watch and Stork™ baby monitor, as well as sound devices from legendary audio brands like Bowers & Wilkins® and Denon®.
Founded by Joe Kiani in 1989 as a private "garage start-up" company, today Masimo is publicly traded and employs more than 8,000 people worldwide. Licensing agreements allow Masimo medical technologies to work inside monitoring devices from a host of manufacturers, including Philips, Atom, Mindray North America, GE Medical, Spacelabs, and Zoll. Throughout its more than 30-year journey, Masimo’s mission – to improve patient outcomes, reduce the cost of care, take noninvasive monitoring to new sites and applications, and improve life – has remained strong. In 2022, Masimo acquired Sound United, a leading developer of premium consumer sound and home integration technologies, adding to its broad portfolio of hospital and home medical technology and wellness solutions.
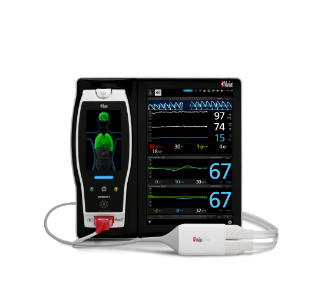
SET® Pulse Oximetry
Joe Kiani started Masimo because he was convinced that the use of adaptive signal processing in the measurement of physiological parameters could solve the problems of low perfusion and motion, which had long plagued in-vivo monitoring and especially pulse oximetry, the measurement of the oxygen saturation of arterial blood. The solution, Masimo SET® Measure-through Motion* and Low Perfusion™ pulse oximetry, has been shown in more than 100 independent and objective studies to outperform other pulse oximetry technologies.1 Masimo SET® measures accurately across all patient populations and clinical settings, including through motion, during low perfusion, and on all skin tones − with multiple studies finding no clinically significant differences in SpO2 bias between individuals with dark and light skin.2-5 Today, Masimo SET® helps clinicians monitor more than 200 million patients in healthcare settings around the world each year,6 and is the primary pulse oximetry technology at all ten of the top 10 hospitals as ranked in the 2024-2025 Newsweek Best Hospitals listing.7
* Masimo SET® Measure-through Motion technology includes SpO2 and PR.
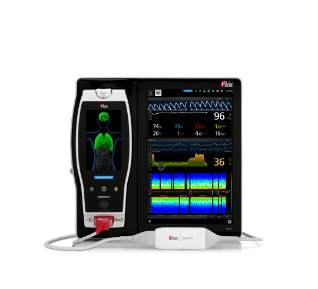
Masimo rainbow® Technology
rainbow®, introduced in 2005, allows additional parameters to be noninvasively and continuously monitored. Advanced signal processing algorithms and unique adaptive filters work together to noninvasively isolate, identify, and quantify various types of hemoglobin, including total hemoglobin, Masimo SpHb®.
rainbow SET® measurements include:
Perfusion Index
Respiration Rate from the Pleth
Sensors and Sustainability
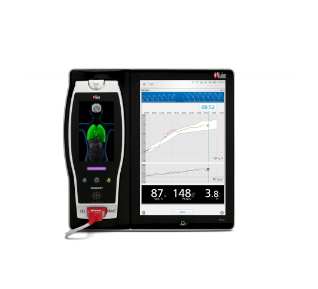
Powered by breakthrough Masimo technologies like SET® pulse oximetry, rainbow® Pulse CO-Oximetry, rainbow Acoustic Monitoring®, and more, Masimo sensors are available in a wide variety of designs to serve many types of patients and care scenarios. Recent innovations include the RD family of sensors – with industry-leading SpO2 accuracy specifications – and tetherless Radius PPG® pulse oximetry.
Masimo offers recycling solutions for all Masimo pulse oximetry sensors, as part of our dual commitment to excellent patient care and environmental responsibility. By recycling sensors with Masimo instead of reprocessing through a third party, our customers help contribute to the goal of zero waste to landfill while maintaining peace of mind that all sensors meet our clinical performance standards.
Versatile Medical Devices
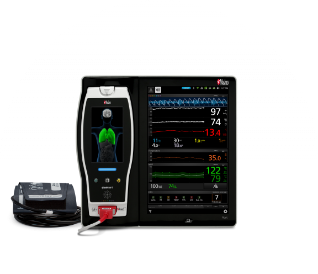
Masimo SET® and rainbow® technologies, collectively known as rainbow SET, fuel medical monitoring devices such as Root®, Radical-7®, Radius VSM™, Radius-7®, Rad-97®, Rad-G® with Temperature, and Masimo W1 Medical. These highly versatile devices are configurable for use in a variety of high- and low-acuity hospital settings, from ICUs and surgical suites to postoperative floors, as well as in long-term care facilities and in the case of Rad-97 and Masimo W1, home use.
Complementing devices that primarily monitor continuously, Masimo spot-check devices such as Rad-67®, MightySat® Rx, and Rad-G allow physician offices, EMS responders, and others to measure oxygen saturation and other valuable measurements.
Expanding Monitoring with Root and Radius VSM™
Masimo continues to expand what can be monitored and how. Root, for example, was built from the ground up to be as flexible and expandable as possible, with Iris® ports and Masimo Open Connect® (MOC-9®) to facilitate the addition of other Masimo and third-party monitoring technologies. Key Masimo Root technologies include:
Next Generation SedLine® Brain Function Monitoring
Noninvasive Blood Pressure and Temperature
Pathway
O3® Regional Oximetry
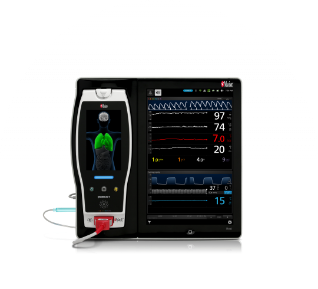
NomoLine® Capnography Solutions
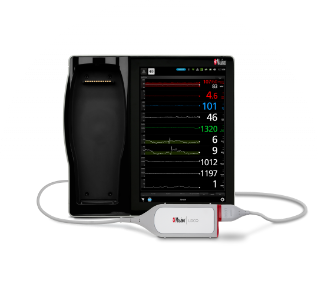
LiDCO® Hemodynamic Monitoring
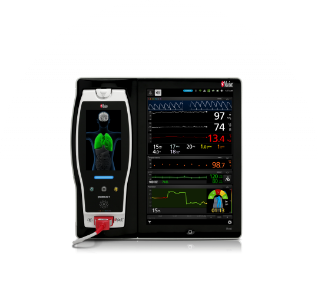
Vital Signs Check
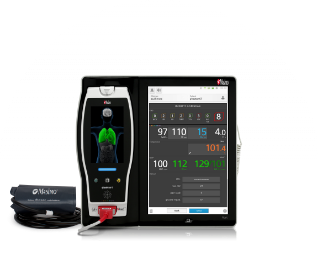
Centroid®
Designed for use across the continuum of care, Radius VSM, a patient-worn, tetherless, continuous vital signs monitor, is similarly versatile and flexible—easily scalable to accommodate surges in patient volume and to suit each patient’s monitoring needs and level of acuity. Radius VSM’s modularity allows it to monitor any combination of SpO2, NIBP, ECG, continuous temperature, acoustic respiration rate, and patient orientation, position, and activity monitoring.
Masimo Hospital Automation™
Driven by Masimo's expertise in artificial intelligence, the Hospital Automation platform offers a broad array of connectivity and automation solutions designed to help clinicians proactively spot possible patient deterioration sooner.
- Halo ION® aggregates trend data from multiple physiologic parameters into a single, continuous score for each patient, configured according to hospital policy and displayed and dynamically updated at Patient SafetyNet central view stations and on Replica devices.
- Masimo iSirona™ and Iris® Gateway gather medical data from devices throughout the hospital, including bedside Masimo and third-party devices and ADT systems, and convert and transfer them to a central repository, the EMR, automating clinician access to a more complete picture of each patient.
- To those abilities, Masimo Patient SafetyNet™ with Replica® adds hospital-wide remote monitoring, robust telehealth capabilities with multi-way video conferencing, and two-way remote clinician notification from a central server, customizable view stations, and mobile devices.
Masimo also offers solutions designed to help care teams remotely manage large quantities of low-acuity patients—for example during the COVID-19 surge in patient volume—with the Masimo SafetyNet® platform.
- Masimo SafetyNet is an economically scalable, cloud-based patient management telemedicine and telehealth solution that combines SET® Radius PPG tetherless pulse oximetry and continuous wireless Radius Tº® temperature measurement (with the ability to integrate numerous additional monitoring devices), with a remote data capture and surveillance platform, accessible from the patient’s smartphone and a web-based clinician dashboard.
- Clinicians can also create, relay, and manage treatment plans for more than 150 types of care, including COPD, heart failure, and oncology, as well as schedule and conduct multi-way video consultations with patients—making Masimo SafetyNet a complete end-to-end home care solution.
† The use of the trademark PATIENT SAFETYNET is under license from University HealthSystem Consortium.
Opioid Safety
Opioid overdose is the leading cause of accidental death in the US, responsible for more than 80,000 of the approximately 100,000 drug-related deaths in 2021, from both illicit opioids and prescription opioids.4 More than 143 million prescriptions for opioids were written in the U.S. in 2020 alone.5 Masimo is committed to expanding innovation solutions beyond the hospital, empowering everyday people and clinicians alike with tools specific to fighting the opioid epidemic.
Opioid Halo™ is the first and only FDA-cleared monitoring solution for detecting opioid-induced respiratory depression, the leading cause of death from opioid overdose.
Bridge™ is a drug-free opioid withdrawal device, the first FDA-cleared device of its kind, designed to help reduce the symptoms of opioid withdrawal.
Corporate Headquarters
Masimo Corporation
52 Discovery
Irvine, CA 92618
(949) 297-7000
References:
- 1.
Estimate: Masimo data on file.
- 2.
3. http://health.usnews.com/health-care/best-hospitals/articles/best-hospitals-honor-roll-and-overview.
- 3.
Shah N et al. J Clin Anesth. 2012 Aug;24(5):385-91.
** The use of the trademark PATIENT SAFETYNET is under license from University HealthSystem Consortium.
PLCO-003013/PLM-11396B-1223