Home / Acoustic Respiration Rate (RRa®)
Acoustic Respiration Rate (RRa®)
rainbow Acoustic Monitoring®
rainbow Acoustic Monitoring®
Acoustic Respiration Rate (RRa®) provides noninvasive and continuous monitoring of respiration rate for all patient populations

- The respiratory signal is separated and processed using acoustic signal processing that leverages Masimo Signal Extraction Technology (SET®) to display continuous respiration rate (RRa) and an acoustic respiration waveform, a visualization of the vibrations caused by the patient’s airflow.
- Clinicians have the option to use the acoustic sensor to listen to the sound of a patient’s breathing remotely from a Masimo Patient SafetyNet™* view station.
- An adhesive Respiratory Acoustic Sensor (RAS) detects acoustic signals produced by the turbulent airflow in the upper airway that occurs during inhalation and exhalation, while signal processing algorithms convert the acoustic patterns into breath cycles to calculate respiration rate.
- Continuous monitoring of SpO2 and RRa, as well as other physiologic parameters, on a single Masimo Pulse CO-Oximeter® provides clinicians with more data to make informed care decisions and helps facilitate well rounded patient assessment.
The Need for Continuous Respiration Monitoring
The Need for Continuous Respiration Monitoring
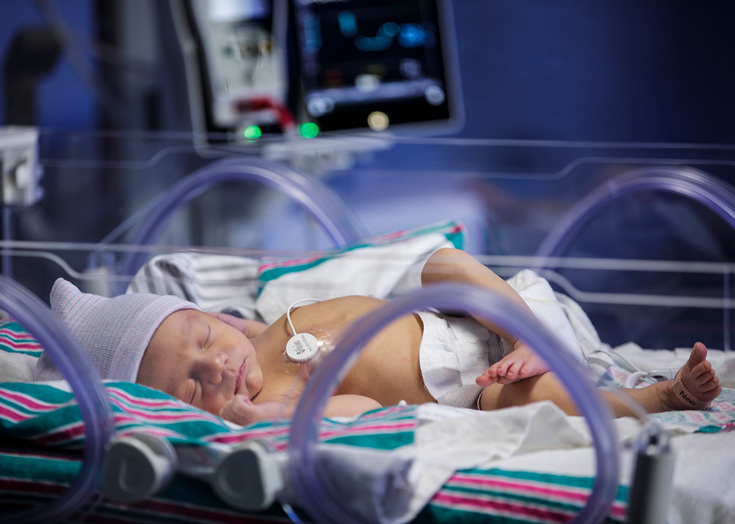
Neonatal intensive care unit (NICU) patients (neonates) need continuous monitoring of vital signs such as respiration rate without causing discomfort or irritation.1 Respiratory conditions are the most common reason for admission to a neonatal unit in both term and preterm infants.2 Respiratory rate (RR) is one of the most sensitive markers of patient condition and a core aspect of multiple clinical assessment tools.3
Pediatric Patient Tolerance
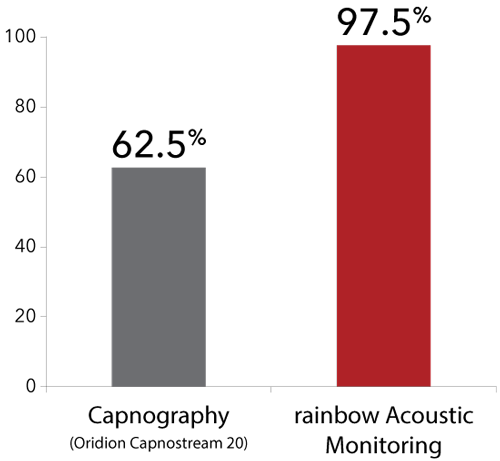
15 out of 40 pediatric patients removed the nasal cannula while only one removed the rainbow® acoustic sensor.
In a study of 40 pediatric patients (12 months to 18 years of age) undergoing post-anesthesia care, in which researchers compared acoustic respiration rate monitoring using RRa to nasal capnography, impedance pneumography, and a reference method (counting breaths), researchers found that the difference in bias and precision between RRa and capnography was not significant, but that 97.5% of the patients (39) demonstrated good tolerance of the acoustic sensor, whereas 62.5% (25) demonstrated good tolerance of the nasal cannula. The researchers concluded, “Continuous respiration rate measurement from noninvasive, acoustic monitoring showed good agreement with nasal capnography, but was much better tolerated in post-surgical pediatric patients. Acoustic monitoring has the potential to increase the safety of pediatric patients by providing a reliable and accurate method for the continuous monitoring of respiration rate.”4
Respiratory Acoustic Sensors (RAS)
Respiratory Acoustic Sensors (RAS)
For All Patient Populations - Adult, Pediatric, Infant, and Neonatal

RAS-45
Adult — Small size with thin, flexible adhesive allows for comfortable application on patients with smaller necks or fragile skin
Infant/Neonatal — Small size with a chest application site away from the face allows for continuous respiratory rate monitoring without interfering with daily care activities, such as feeding, holding, bathing, and supine positioning

RAS-125c
Adult/Pediatric — Breathable cloth allows air to penetrate tape for enhanced adhesion on adult and pediatric patients, including diaphoretic patients
rainbow Acoustic Monitoring® Sensors and Cables
Explore the rainbow Acoustic Monitoring line of Sensors & Cables
References:
- 1.
Abbas et al. BioMedical Engineering OnLine. 2011, 10:93.
- 2.
Pramanik AK et al. Pediatr Clin North Am. 2015; 62: 453 -469.
- 3.
Keir et al. J Clin Monit Comput. (2015) 29:455 -460. DOI 10.1007/s10877-014-9621-3.
- 4.
Patino M et al. Pediatric Anesthesia. 2013, no. 12: 1166-1173.
*The use of the trademark PATIENT SAFETYNET is under license from University HealthSystem Consortium.
RESOURCES
For professional use. See instructions for use for full prescribing information including indications, contraindications, warnings and precautions. Caution: Federal (USA) law restricts this device to sale by or on the order of a physician.
PLCO-003388/PLM-11608C-0221