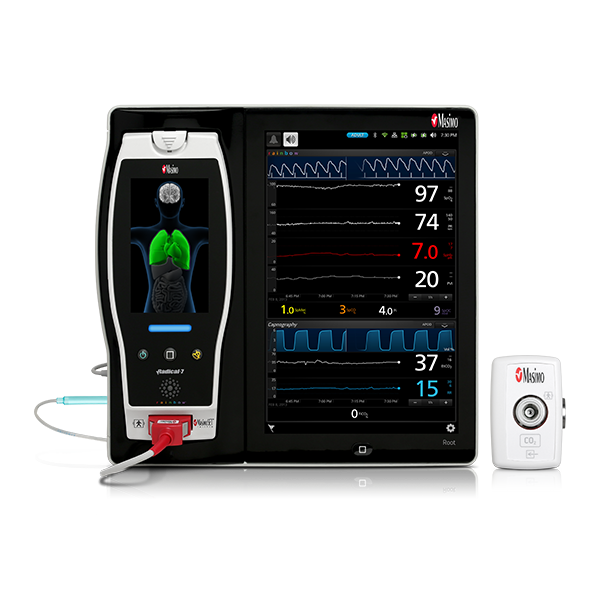
Respiratory Care
Assess. Protect. Maintain. Improve.
Home / Respiratory Care
Respiratory Care
Monitoring oxygenation and ventilation throughout the breathing cycle helps provide a more complete picture of a patient's respiratory status.
Oxygenation
Oxygenation
Masimo’s breakthrough technology, Signal Extraction Technology® (SET®), overcame the limitations of conventional pulse oximetry with the ability to measure through motion and low perfusion. Masimo SET® is able to recognize that both arterial and venous blood can move, using parallel signal processing engines to separate the true arterial signal from sources of noise, including the venous signal. Multiple studies have demonstrated that Masimo SET® outperforms conventional pulse oximetry technologies.1 In one study comparing the ability of three pulse oximetry technologies to detect hypoxic events, Masimo SET® pulse oximetry demonstrated the highest sensitivity and specificity during induced conditions of motion and low perfusion.2
Performance During Motion and Low Perfusion2
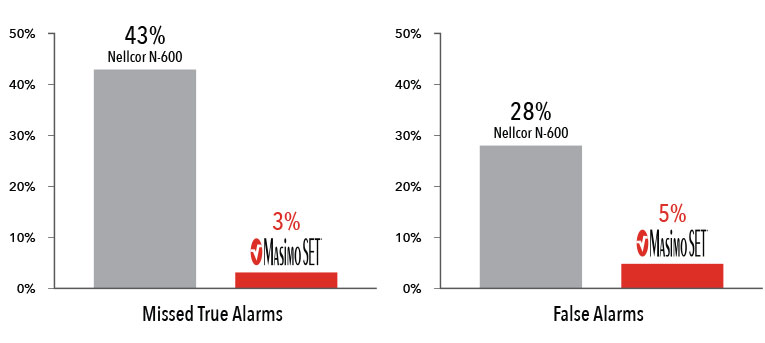
Masimo SET® had 3% missed true alarms and 5% false alarms versus 43% and 28%, respectively, using competitor technology.2
Results shown are calculated by combining sensitivity and specificity outcomes of the machine-generated and volunteer-generated motion.
At Masimo, innovation never stops. After these studies were published, Masimo achieved significant accuracy specification improvements for RD SET® sensors, improving from 3% ARMS to 1.5% ARMS in conditions of motion.*
While Masimo pulse oximeters offer SET® SpO2 and pulse rate (PR), among other measurements, Masimo Pulse CO-Oximeters® offer additional advanced measurements, including total haemoglobin (SpHb®) and dyshaemoglobins, expanding visibility of a patient’s oxygenation status.
Ventilation
Ventilation
Capnography is a noninvasive monitoring technique that allows fast and reliable insight into ventilation, circulation, and metabolism.
In situations requiring intubation, capnography has long played a role in confirming proper endotracheal tube placement and, in both elective and emergency intubations, end-tidal carbon dioxide (EtCO2) measurement is a standard of care for confirming endotracheal intubation.3 In addition, EtCO2 provides a noninvasive estimate of the partial pressure of carbon dioxide (PaCO2) and insight into a patient’s breathing rate and pattern before extubation.3
When used together, capnography and pulse oximetry are useful in weaning patients off mechanical ventilation.4 In addition, capnography also facilitates monitoring of spontaneous ventilation after weaning and during sedation procedures.4,5 A 2017 meta-analysis of sedation-related adverse events found that the inclusion of capnography monitoring resulted in a reduction in respiratory compromise during procedural sedation and analgesia compared to visual assessment and SpO2 alone.6
NomoLine® Capnography eliminates common problems associated with conventional sidestream gas analysis. Incorporating a unique, patented polymer, NomoLine sampling lines allow water to evaporate into the surrounding air, while leaving oxygen, carbon dioxide, and anaesthetic gases unaffected, eliminating the need for a water trap and issues related to their handling. Designed for low-flow applications, with functionality in any orientation, NomoLine sampling lines can be used in a variety of clinical scenarios on both intubated and non-intubated adult, pediatric, infant, and neonatal patients, in both low- and high-humidity applications.
Innovations in Respiration Rate Monitoring: rainbow Acoustic Monitoring®
rainbow Acoustic Monitoring provides a noninvasive, continuous, easy-to-use, and reliable monitoring solution that has the benefit of higher patient compliance.7,8 Additionally, continuous monitoring of SpO2 and acoustic respiration rate (RRa®), as well as other physiologic parameters, on a single Masimo Pulse CO-Oximeter facilitates well-rounded patient assessment and provides clinicians with more data to make informed care decisions.
How it Works
A Respiratory Acoustic Sensor, such as the RAS-45 or RAS-125c, detects acoustic signals produced by the turbulent airflow in the upper airway that occurs during inhalation and exhalation, while signal processing algorithms convert the acoustic patterns into breath cycles to calculate respiratory rate. The respiratory signal is separated and processed to display continuous RRa measurements and waveforms, with the option to listen to the sound of breathing from the acoustic sensor.

Masimo Portfolio
Masimo Portfolio
Masimo offers a complete portfolio of oxygenation and capnography solutions with industry-leading pulse oximetry and CO-Oximetry technologies and both sidestream and mainstream capnography options. These solutions meet the challenges of alveolar ventilation monitoring in virtually all care areas, including pre-hospital, in-hospital, transport, long-term care, ambulatory care centers and clinics, physician and dental offices, and home care. Solutions range from integrated OEM offerings, to external “plug in and measure” gas analyzers, to bedside and handheld devices.
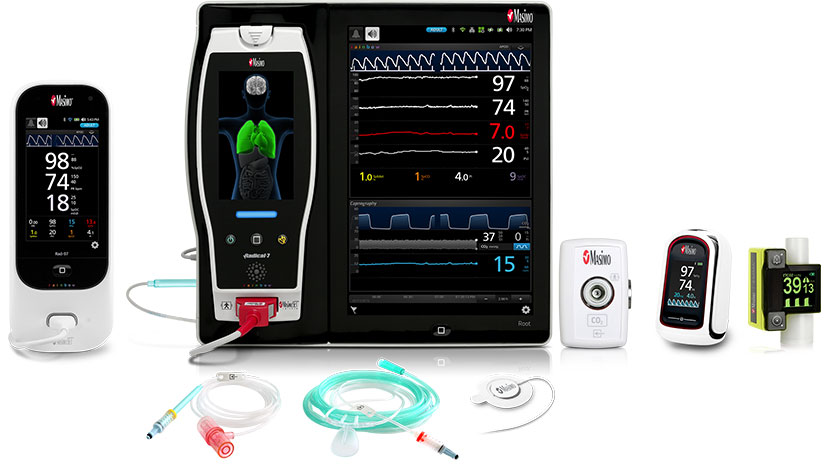
Rad-97, Root® with ISA™ CO2, MightySat™ Rx, EMMA™ Capnograph, NomoLine Sampling Lines, RAS-45 Sensor
Measurements
Measurements
Respiratory Care Product Solutions
References:
- 1.
Masimo. Clinical Evidence. https://www.masimo.com/evidence/featured-studies/feature/.
- 2.
Shah et al. J Clin Anesth. 2012;24(5):385-91.
- 3.
Nagler J et al. Emerg Med Clin North Am. 2008 Nov;26(4):881-97.
- 4.
Capnography in Pediatrics. Bhavani Shankar Kodali MD. http://www.capnography.com/pediatrics. Sourced January 2018.
- 5.
Canography in Intensive Care Unit. Samuel M. Galvagno Jr., D.O., Bhavani Shankar Kodali, M.D.www.capnography.com. http://www.capnography.com/icu/capnography-in-icu sourced in January 2018
- 6.
Saunders, R., Struys, M. M. R. F., Pollock, R. F., Mestek, M., & Lightdale, J. R. (2017). Patient safety during procedural sedation using capnography monitoring: A systematic review and meta-analysis. BMJ Open, 7(6) doi:http://dx.doi.org/10.1136/bmjopen-2016-013402)
- 7.
Macknet MR et al. Accuracy and Tolerance of a Novel Bioacoustic Respiratory Sensor in Pediatric Patients. Anesthesiology. 2007;107:A84. (abstract).
- 8.
Goudra BG et al. Comparison of Acoustic Respiration Rate, Impedance Pneumography and Capnometry Monitors for Respiration Rate Accuracy and Apnea Detection during GI Endoscopy Anesthesia. Open J Anesthesiol. 2013; 3:74-79
*ARMS accuracy is a statistical calculation of the difference between device measurements and reference measurements. Approximately two-thirds of the device measurements fell within +/- ARMS of the reference measurements in a controlled study.
RESOURCES
For professional use. See instructions for use for full prescribing information, including indications, contraindications, warnings, and precautions.
PLCO-004333/PLM-11361B-0920